Simplicity and user-friendliness are vital, and cannot be emphasised plenty of. It must be attainable to locate certain sections/paperwork quite a few years later on and the provider need to take into account if the composition is reasonable. If it seems sophisticated it ought to be improved until eventually it can be stated and described in a very reasonable method.
For compendial compliance, the water Evaluation needs to be representative of your water Utilized in output. Normally, the web instruments are located downstream of the final outlet, and never following any with the purification device functions in the distribution system.
Distillation is helpful in generating WFI that meets the demanding microbial and endotoxin specifications set by pharmacopeias.
Purifying water for dialysis devices contributes to 25 litres of water remaining wasted for each moment, according to the healthcare facility.
While using the additional proven technique of distillation, this capabilities by turning water from the liquid to your vapour and afterwards from vapour back again to liquid. Endotoxin is taken off by the speedy boiling which leads to the water molecules to evaporate and also the fairly much larger endotoxin advanced molecules to remain behind.
Development from the hydraulic containment system commenced April ten alongside the base’s western fence read more line by Züblin, a Stuttgart-based mostly building and civil engineering firm, a decade once the “for good chemical compounds” have been uncovered.
Purified water (PW) is Utilized in the pharmaceutical field being a raw content in production or to scrub tools. It truly is, hence, crucial which the water fulfills the established criteria and continuously presents the required high quality and amount to be sure there is not any contamination from the products or products.
Hence, a suitably managed signifies of planning, storage and distribution should be employed to make certain that the limits are complied with at stage of use.
It shows that an illustration needs to be performed for the reliable production click here and shipping of water of your necessary amount and top quality. If the system is operated According to the common working technique.
Offer rigorous testing to reveal the performance and reproducibility of the entire built-in course of action.
Qualification action shall take into account phases from your initial enhancement with the user need specification through to the top of using the machines, facility, utility, or system.
At the end of the task, when all inspections and tests are carried out and probable deviations are calculated, it is crucial which the 'as crafted' layout documents are included in to the documentation with the system (Figure 1).
Warn and Action Levels are system Command terms and may be proven at ranges indicative with the water system trending outside of its standard microbial Regulate assortment. These ranges really should be set up at stages no increased than, and if possible decrease than, All those detailed in
So, If you're establishing a brand new facility, you'll unquestionably want help with water system validation. so for that validation authority ought to be authorized by state drug control and CDSCO, underneath the Medicine and Cosmetics Act.
 Kelly McGillis Then & Now!
Kelly McGillis Then & Now!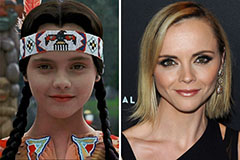 Christina Ricci Then & Now!
Christina Ricci Then & Now! Melissa Joan Hart Then & Now!
Melissa Joan Hart Then & Now! Elisabeth Shue Then & Now!
Elisabeth Shue Then & Now! Samantha Fox Then & Now!
Samantha Fox Then & Now!